Quality Control in Pharma Production
To release drug samples, producers have to fullfill severell steps in quality control. For this purpose, Kapelan offers a sophisticated system to document all steps. With the help of our FDA 21 CFR Part 11 compliance a gap-free traceability and proper archiving of your data is guaranteed.
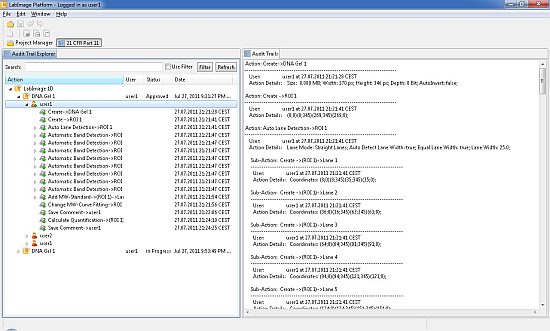
The 21 CFR Part 11 compliant modules consist of 3 main segments:
- user accounting with role management
- document each step in Audit Trails
- secure data storage
The Part 11 compliant modules are part of the LabImage software and can be used for each app. Read more on the 21 CFR Part 11 Compliance as module in LabImage. LabImage delivers modules for device controling as well to create a closed system.
More Solutions
- Multi Channel Western Blots with Smart Protein Layers Technology
- Quality Control in Pharma Production
- Strip Analysis and Line Assays
- LabImage 1D in EPO diagnostics
- LabImage used in Scientific Papers
- Extensions in LabImage 1D- a valuable addition to the key functions
- Grayscale Chart Calibration Service
- Apps in Medical Image Processing
- Consulting for FDA 21 CFR Part 11 and ISO 13485
